Electrochemical Impedance Spectroscopy (EIS) is a powerful, non-destructive technique used to analyze the internal properties of batteries by applying an AC signal over a wide frequency range and measuring the cell’s response. EIS helps diagnose battery health, aging, and performance by modeling impedance versus frequency, offering critical insights for battery research and management.
What is Electrochemical Impedance Spectroscopy (EIS) and how does it work?
EIS is an electrochemical measurement method that applies a small AC voltage over a range of frequencies to a battery or electrochemical cell and measures the resulting current response. It determines a cell’s impedance spectrum, revealing resistive and capacitive elements that correspond to physical and chemical processes inside the battery.
By analyzing impedance at different frequencies, EIS isolates contributions from electrolyte resistance, solid electrolyte interphase (SEI) layers, charge transfer reactions, and diffusion phenomena, enabling precise diagnostics of battery condition.
Wholesale lithium golf cart batteries with 10-year life? Check here.
EIS is widely used in lithium-ion battery research, including at Redway Battery, to facilitate real-time, non-invasive monitoring of battery State of Health (SOH) and performance optimization.
How is EIS data represented and interpreted in battery testing?
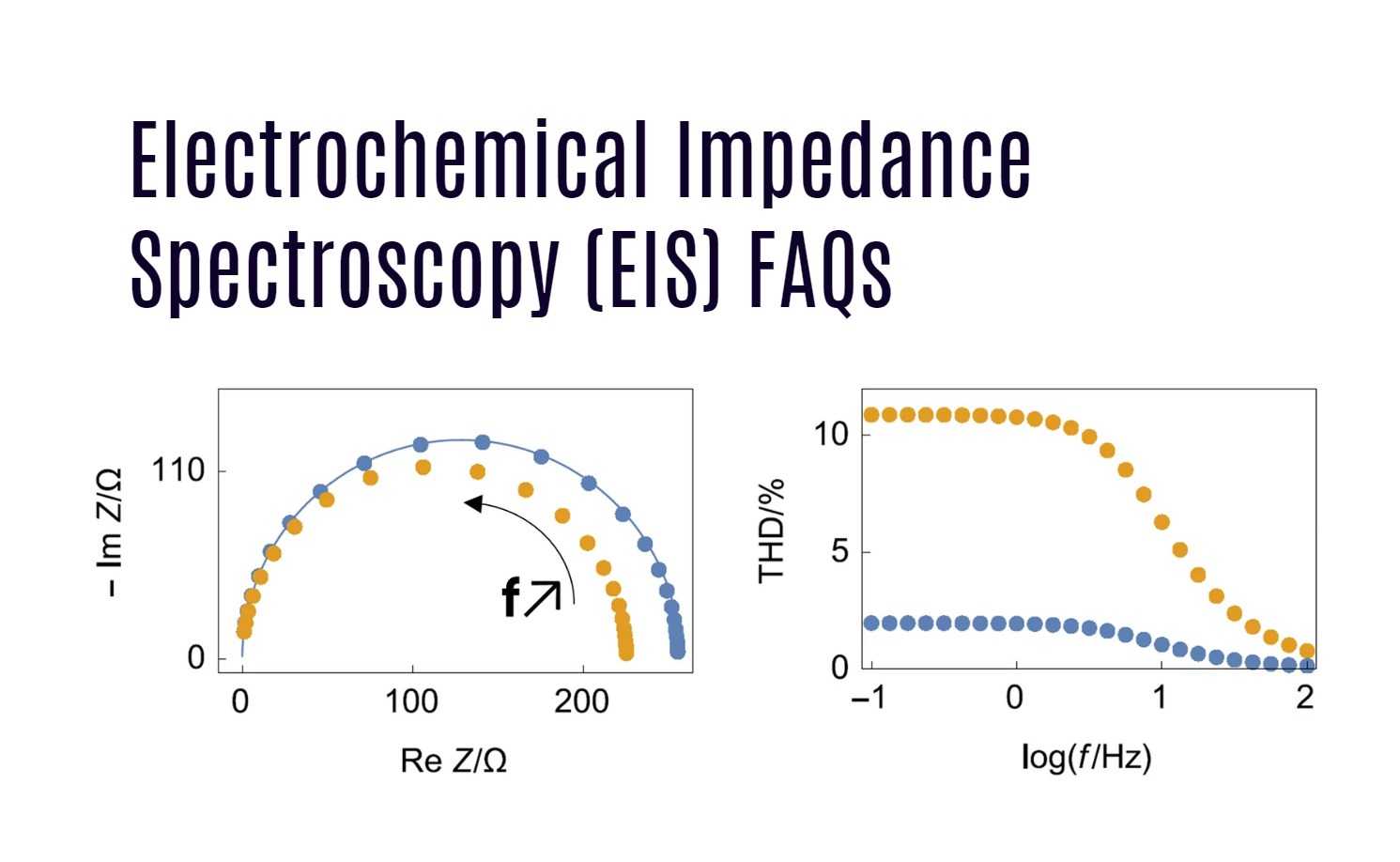
EIS results are typically plotted in Nyquist or Bode plots:
Want OEM lithium forklift batteries at wholesale prices? Check here.
-
Nyquist plots show the imaginary part of impedance versus the real part, often creating semicircles and lines that correspond to different electrochemical processes.
-
Bode plots display impedance magnitude and phase angle across frequencies, helping interpret kinetics and resistances.
From these plots, battery parameters like bulk resistance (electrolyte resistance), charge transfer resistance, and double-layer capacitance are extracted by fitting the data to equivalent circuit models that represent physical battery components.
Proper interpretation requires advanced fitting algorithms and domain expertise, frequently integrated into Redway Battery’s quality control to ensure battery reliability.
Which battery parameters can EIS measure effectively?
EIS can measure multiple vital parameters such as:
-
Bulk resistance (R_b): Resistance of electrode materials, electrolyte, and separator.
-
Charge transfer resistance (R_ct): Resistance to electrochemical reactions at electrode interfaces.
-
Solid Electrolyte Interphase (SEI) layer resistance and capacitance: Indicators of surface film quality.
-
Diffusion coefficients: Reflect lithium-ion transport within electrodes.
-
State of Health (SOH): Changes in impedance correlate with battery aging and capacity loss.
These measurements provide crucial data for optimizing battery design, diagnosing degradation modes, and improving safety and longevity.
Why is EIS considered a non-destructive testing method for batteries?
EIS uses very small AC signals that do not significantly alter the battery’s chemical state or cause damage. It can be performed during battery idling or cycling without disassembly, providing insights into internal processes without degrading performance.
This non-invasive nature enables frequent monitoring and real-time assessment, a key advantage leveraged by Redway Battery in their manufacturing and quality assurance processes.
When is EIS testing most beneficial during a battery’s lifecycle?
EIS is valuable during all stages but especially:
-
In research & development for characterizing new battery chemistries and architectures.
-
During manufacturing and quality control to ensure parameter consistency and detect defects.
-
Throughout battery cycling to track aging, SOH, and performance degradation.
-
In predictive maintenance for electric vehicles and energy storage systems to prevent failures.
Redway Battery uses EIS regularly for R&D and production validation to maintain high-quality energy storage solutions.
How does temperature affect Electrochemical Impedance Spectroscopy measurements?
Temperature influences battery kinetics and impedance elements. Elevated temperatures typically reduce charge transfer resistance and diffusion impedance but can accelerate degradation. Low temperatures increase resistance and slow reactions.
EIS measurements should account for temperature variations to accurately interpret results. Redway Battery’s testing protocols integrate temperature control to ensure unbiased impedance analysis.
Can EIS be used for real-time battery management systems?
While EIS offers detailed diagnostics, traditional single-frequency or slow multi-frequency EIS techniques require significant measurement time. However, new rapid EIS methods using pseudo-random sequences and advanced signal processing are emerging, aiming to integrate EIS into real-time Battery Management Systems (BMS) for continuous SOH monitoring.
Redway Battery continuously explores such innovations, enhancing their battery packs with intelligent monitoring capabilities.
What equipment and software are required for EIS analysis?
EIS requires potentiostats or impedance analyzers capable of generating AC signals and measuring current response over a frequency range, from mHz to kHz. Data are then processed using specialized software for equivalent circuit fitting and parameter extraction.
Sophisticated EIS tools enable accurate battery modeling and insights, critical to Redway Battery’s development and customization of lithium battery packs.
Does EIS help in identifying specific battery failure mechanisms?
Yes. By analyzing impedance spectra, EIS can detect:
-
Increased SEI resistance indicating electrolyte decomposition.
-
Rising charge transfer resistance due to electrode surface degradation.
-
Changes in diffusion impedance revealing loss of active material.
-
Internal short circuits or connection faults shown by abnormal impedance features.
These insights allow targeted interventions and design improvements, extending battery lifespan.
Are there limitations or challenges in applying EIS to commercial battery systems?
Challenges include:
-
The need for accurate equivalent circuit models customized to each battery design.
-
Long measurement times limiting applicability in fast-paced environments.
-
Complexity in interpreting overlapping impedance features in aged or multi-material batteries.
-
Sensitivity to measurement conditions such as temperature and state-of-charge.
Research efforts including by Redway Battery aim to overcome these by developing faster EIS techniques and robust analytical models for practical applications.
Comparison Table: Key Electrochemical Impedance Components in Battery Analysis
| Component | Frequency Range | Represents | Typical Impact on Battery |
|---|---|---|---|
| Bulk Resistance (R_b) | High frequencies | Electrolyte and separator | Increased internal resistance |
| SEI Layer Resistance (R_SEI) | Mid frequencies | Solid electrolyte interphase layer | Degradation indication |
| Charge Transfer Resistance (R_ct) | Mid to low frequencies | Electrochemical reaction kinetics | Slower kinetics, aging indicator |
| Diffusion Impedance (Warburg) | Low frequencies | Lithium-ion diffusion | Limited ion transport, capacity loss |
Redway Expert Views
“At Redway Battery, Electrochemical Impedance Spectroscopy is an indispensable tool in our pursuit of high-performance, reliable lithium battery solutions. Leveraging EIS allows us to characterize internal battery phenomena accurately, identify degradation early, and support tailored engineering enhancements. This depth of insight ensures our battery packs meet rigorous safety and longevity standards demanded by electric vehicles, forklifts, and energy storage markets worldwide.” — Redway Battery Engineering Team
Conclusion
Electrochemical Impedance Spectroscopy is a sophisticated and non-destructive analytical technique critical for understanding battery internal processes, health, and aging. By dissecting complex electrochemical phenomena across frequencies, EIS provides invaluable diagnostics used in research, manufacturing, and real-time monitoring. Despite challenges in modeling and measurement speed, advancements continue to improve its practicality. Integrating EIS in battery management, as employed by industry leaders like Redway Battery, enhances performance, reliability, and safety in lithium battery technologies.
Frequently Asked Questions
Q1: Can EIS be performed on a fully charged battery?
A1: Yes, EIS can be performed at different states of charge, but steady-state conditions provide the most accurate data.
Q2: How long does an EIS measurement typically take?
A2: Conventional EIS tests can take from minutes to hours depending on frequency range and resolution; faster methods are emerging.
Q3: Does EIS require special battery preparation?
A3: Batteries should be in a stable condition, ideally at rest, with controlled temperature for consistent results.
Q4: Is EIS suitable for all battery chemistries?
A4: Yes, EIS is widely applicable but equivalent circuit models must be tailored to specific chemistries for best results.
Q5: How does Redway Battery use EIS in product development?
A5: Redway employs EIS for quality control, aging studies, and optimizing battery pack design to ensure longevity and safe operation.